Cleaning, Decontamination, Sterilization
-
Posted: May 12, 2021Read more »
Check out our list of Dos and Don’ts in a cleanroom, a summary of cleanroom best practices for making the controlled environment function at peak performance. These are good resources for new cleanroom operators or those unsure about what steps to take in a cleanroom environment.
-
Posted: April 23, 2020Read more »
Cleaning a laminar flow hood sounds like a self-explanatory task, but there are plenty of factors to consider when the stakes of sterilization include the integrity of your products. Even more importantly, a clean laminar flow hood helps ensure the safety of patients and consumers on the receiving end of those products.
-
Posted: June 05, 2019Read more »
Most cleanroom professionals understand that Fan/Filter Units (FFUs) capture contaminants that degrade particle-sensitive samples. But they also remove bacteria, viruses and mold spores that contribute to a host of infections.
With the threat of superbugs on the rise in medical facilities, sterilization has never been more crucial. Superbugs, or drug-resistant bacteria that cannot be killed by standard antibiotics, have the potential to cause infections that are increasingly difficult to cure. The number of deaths related to these bacteria is decreasing. But the bugs, and the danger, remain present.
Until new cures are developed to battle bacteria, the solution to preventing the spread of germs is to update cleanliness procedures in hospitals, pharmaceutical manufacturing facilities and other places where drugs are regularly developed or used. Hand-washing can push back against the threat, but it’s important to implement protective measures against any potential breach,
-
Posted: January 01, 2019Categories: Cleaning, Decontamination, SterilizationRead more »
Manufacturers invest hundreds—even thousands—of dollars per square foot of cleanroom space to meet ISO-proscribed particle counts. Shouldn't the same standards be required of the people who enter and potentially contaminate this ultra-clean environment?
Proper cleanroom garments, including hoods, face masks, booties and gloves, help to contain particles that people emit. Yet improper gowning procedures can negate your investment in cleanliness and threaten yields of sensitive semiconductor devices. Once a garment is contaminated—violated by contact with a dirty surface—it spreads particles everywhere it goes.
You can train personnel on proper garmenting procedures, but how do you guarantee compliance? A violated garment doesn't set off alarms, and few facilities can afford quality control monitors to supervise every person through every washing and dressing stage. Yet if strict controls are not observed,
-
Posted: May 12, 2016Read more »
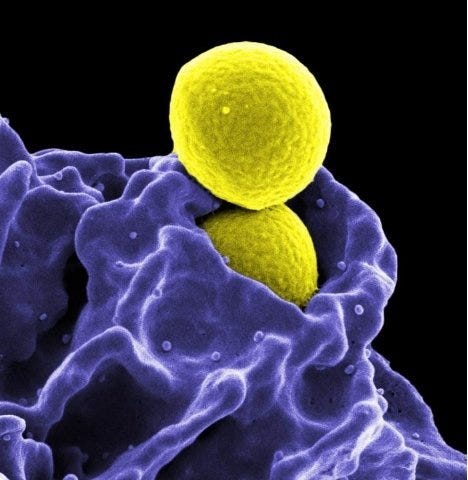
Methicillin-Resistant Staphylococcus aureus (MRSA) bacterium. Photo credit: CDC
Controlling microbial contamination is one of the leading concerns in research, clinical, and medical facilities. Microorganisms (hazardous or not) can put personnel, patients and caregivers at risk. In hospital and medical facilities, patients are often immuno-compromised or have serious conditions that make them particularly susceptible to opportunistic microbes or secondary infections.
For these reasons, many products are available for decontamination of these critical spaces. There are differences in product effectiveness, cost, potential residual damage and operational limitations. Two particular methods, presented here, are commonly employed to reduce or eradicate hazardous microorganisms.
Shining a Light on Microorganisms: UV-C Decontamination
Ultraviolet germicidal irradiation has been a mainstay for killing and inactivating microorganisms
-
Posted: March 24, 2016Read more »
Sterilization is a process designed to destroy and remove all forms of life present in a certain region. It’s accomplished by use of physical or chemical means. Autoclaves, for example, steam sterilize by high pressure and heat (250°F/121°C at the low end).
Another sterilization-by-heating technique uses infrared to kill microorganisms in a few seconds using temperatures up to 1500°F/815°C. On the chemical front, hydrogen peroxide can break down cellular tissue.

BactiZapper™ Infrared sterilizer from Benchmark Scientific
Sterilization can also be used to eliminate
-
Posted: February 25, 2016Read more »
The cleaning and sterilization of laboratory, clinical, surgical and compounding equipment is necessary to ensure patient safety and accurate results. In research laboratories, improperly cleaned or sterilized glassware and instruments opens the door to unwanted contaminants. Contaminated objects can also endanger laboratory personnel through exposure to mutagenic or toxic substances.
In clinical labs, contamination can lead to erroneous test results, jeopardizing patient treatment regimens or diagnoses. In surgical and pharmaceutical facilities, improper sterilization of equipment or compounding preparations can expose patients to a wide range of microbes and endotoxins. Thus, labs must develop and document washing and sterilization protocols that produce reliable results and ensure safety.
According to a Frost and Sullivan survey published in Lab Manager Magazine, 60% of laboratory budgets are spent on consumable products. Another 30% of the total budget is spent
-
Posted: January 25, 2016Read more »
The sun naturally “cleanses” the earth’s surface with ultraviolet energy. Due to conditions like sun burns and skin cancer, we are most familiar with UVA and UVB rays, but there is actually a third classification of ultraviolet energy called UVC. These rays contain the highest energy, making them the most dangerous type of ultraviolet light. Fortunately, Earth’s atmosphere acts as a protective layer and UVC energy does not penetrate our ozone layer. These harmful rays, however, are industrially produced as a beneficial source of UVGI (ultraviolet germicidal irradiation). Keep reading to find out why.
How does UVGI work?
UVGI is a technique that utilizes short-wavelength ultraviolet light to kill microorganisms. Since UVC energy is not naturally present within our planet’s atmosphere, earth-bound microorganisms, such as germs or viruses, have not evolved to naturally defend themselves